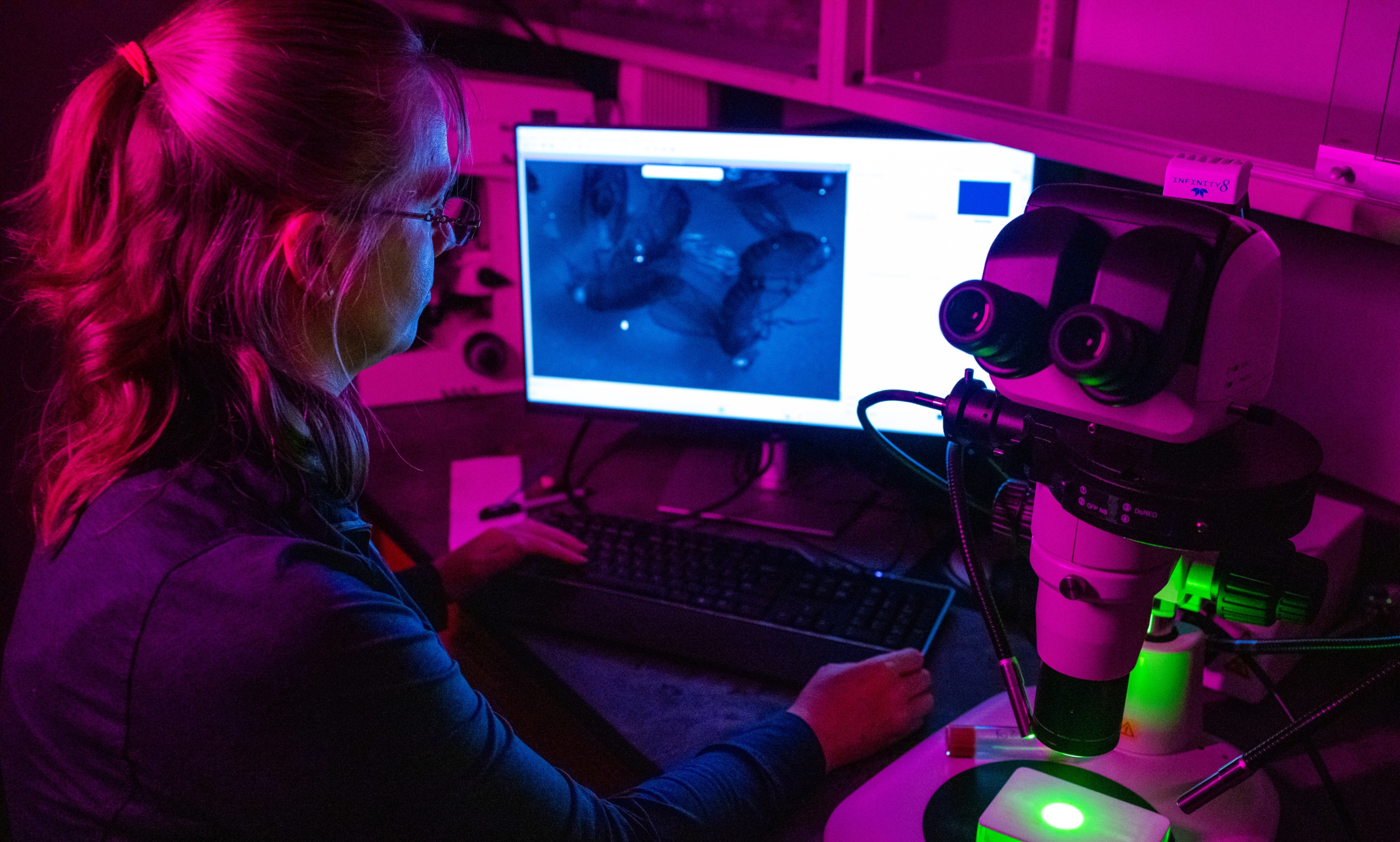
Talk about significant research. UMKC Assistant Professor Maria Spletter’s biology lab is investigating the breakdown of proteins in the body that lead to chronic conditions such as cancer and heart disease. She has lasered in on myotonic dystrophy — or loss of muscle function.
Myotonic dystrophy is among the most common rare diseases, estimated to affect 1 in 2,100 births. The muscle disease also causes accelerated aging as the regulation of ribonucleic acid — present in all living cells and also called “RNA” — changes and alters muscle control, growth and contraction.
Spletter selected a model that is insignificant in size but significant in efficiency: the fruit fly Drosophila.
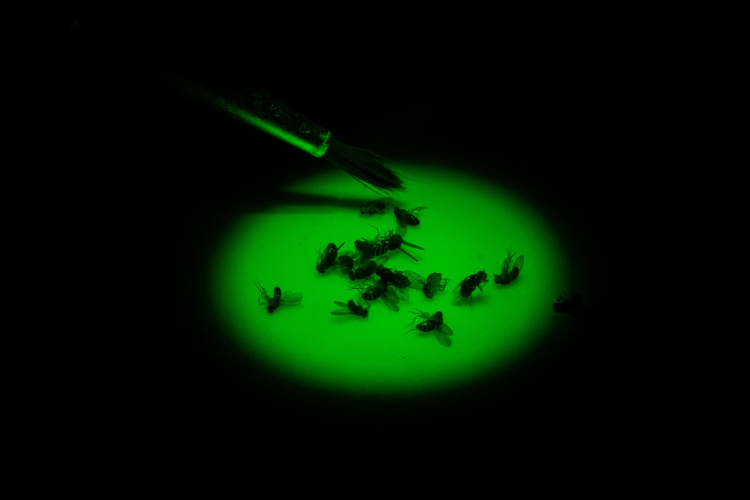
Why Drosophila?
“Drosophila are a very powerful genetic model,” Spletter said. “This means that there are a lot of tools available in the fly that enable us to do experiments that are not possible in mice or rats or humans. Plus, Drosophila grow quickly from an egg to adult fly in 10 days, so you can follow each step during development in a matter of weeks instead of years.”
Another benefit of using Drosophila is the muscles it has are highly conserved, meaning the proteins that build muscles, how muscles contract and the structure and organization of muscles are the same in flies as they are in humans. In fact, models of human disease in flies reveal the same mechanism and same muscle type. These flies, therefore, provide a useful model to understand the basic mechanisms and defects in the muscle that result from a disease-causing mutation, so that further studies in human cells can be targeted.
This means the team can look at the developmental mechanisms that lead to loss or damage to muscle fibers during a time point that is impossible to study in detail with mammals. By tracking the initial stages of muscle fiber during development, they can tell exactly which steps in the assembly process are defective. Studies in mice or rats typically do not have this level of resolution and have not focused in detail on how the structure of the muscle is disrupted.

Testing With High-Powered Tools
“We test muscle function to measure how well flies can fly, jump, climb, flip themselves over after falling on their back and how quickly they are able to clean themselves after being dusted with a fluorescent powder,” Spletter said. “All of these give us insight into live flies on their behavior when it comes to how well their muscles work.”
To investigate the function of these RNA-binding proteins on the cellular level, the lab labels the tip of the muscle with a fluorescent marker and watches the muscle move using a microscope. They then quantify the movement by measuring how often the muscles contract, how much they move when they contract and the dynamics of the contraction. This is where contraction is usually impaired and irregular in mutant flies.
The researchers then use a high-powered microscope that utilizes lasers to image samples to look at muscles that are stained and label different components. Unlike a traditional microscope, the laser allows us to image single planes that are 1 micron or less thick (a fruit fly is about 1 millimeter thick, and there are 1,000 microns in 1 millimeter). An indirect flight muscle cell is around 100 microns thick, so at least 100 pictures of different planes in the muscle can be taken to see all the structures inside it.
With the lab’s microscope having four different lasers, four different components of the cell can be viewed at the same time to see where they are located relative to each other. With the mutants in particular, the team can observe how their localization has changed. This allows comparison between mutant and control flies to see how the structure is different in the mutants on a cellular structural level.
The team then takes tissue samples from control and mutant flies to molecular and biochemical testing to find out what genetically changed in the mutant muscles, subsequently linking molecular defects to changes in cellular structure as well as muscle function.
This is usually when mRNA-Seq, a combination of a biochemistry and bioinformatics approach, takes place. The mRNA (coding blueprints that are turned into proteins in the cell) from the fruit fly is isolated and the lab sequences every single gene and gene variant expressed in the muscle cell. Typically, there are around 6,000 to 8,000 genes expressed at any time, and if you look across development, around 10,000 genes change expression. The data is usually viewed for individual genes, individual splice events within one gene, or globally at all the changes in gene expression and splicing. The various levels of data obtained allow the lab to understand on a systems level what has changed in the mutant muscle cell in comparison to the control, and on an individual gene level to identify targets that might explain specific pieces of the phenotype we see.
Spletter’s lab also conducts mass spectrometry, an analytical tool useful for measuring the mass-to-charge ratio of one or more molecules present in a sample, to isolate the proteins from muscle cells and determine the identity of most of the proteins present in the muscle. Around 4,000 proteins are typically detected, but more sensitive machines can see up to 6,000 proteins. This analytical method provides information on which proteins change in our mutant muscles and allows the comparison of the protein changes in the RNA from the mRNA-Seq data and to find out exactly how changes in RNA regulation lead to defects in muscle fibers and structure.
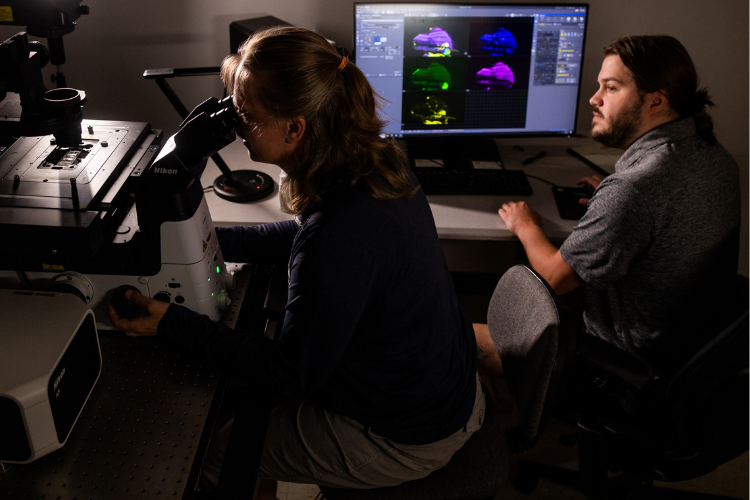
The Discoveries
Recent research findings from Spletter’s lab, which were published on bioRxiv, revealed how the characteristics in mutant muscle are a domino effect. The small things that go wrong at each step in the muscle development lead and further heighten the effect on the muscle. This leads to greater disruption on the muscle, compared to when Bruno1 mutant is added to the later part of muscle development.
From the same research findings, Spletter’s lab discovered the potential possibility of testing gene therapy strategies in the flies that are currently in development for possible use in human patients.
Although gene replacement therapy can “normalize” patterns of splicing, patients only have a partial improvement of symptoms. This means that gene therapy usually improves quality of life, and will likely extend life expectancy, but is not a cure.
Spletter’s lab was able to gain insight into why exactly this is the case.
“Because the structure of the muscle has defects in the core mechanical structure that allows it to move, just fixing the splicing pattern is not sufficient to fix those defects,” Spletter said. “This suggests that we need better detection methods to find patients before they seek medical help, as the earlier a gene therapy can be administered, the better chance these patients are going to have of maintaining muscle function.”